Explain Why Oxygen Is a Fairly Reactive Element
A metal below hydrogen in the reactivity series will not react with dilute acids. 1 on a question.

Atomic Structure And Periodic Table Extra Credit
Learn vocabulary terms and more with flashcards games and other study tools.
. Up to 24 cash back 4 Explain why oxygen is a fairly reactive element while neon is not. View results Why is oxygen a fairly reactive element while neon is not. You do not have to include symbols and atomic numbers.
5 Explain why beryllium loses electrons when forming ionic bonds. Neon has a full outer shell of electrons so theres no particular reason for it to form chemical compounds. Is it harmful or beneficial outcome.
The more reactive the metal the more rapid the reaction is. 3 Explain why oxygen is a fairly reactive element while neon is not. Alkali metals are given the name alkali because the oxides of these metals react with water to form a.
So oxygen will have to share or steal electrons from other elements reactive. Neon has a full outer shell of electrons so theres no particular reason for it to form chemical compounds. Explain why oxygen is a fairly reactive element while neon is not Oxygen has 6 valence electrons and needs two to become stable.
1 explain why oxygen is a fairly reactive element while neon is not2 explain why beryllium loses electrons when forming ionic bonds while sulfur gains electrons3 explain why fluorine and chlorine have similar reactivities hint. Oxygen wants to gain electrons to become like the nearest noble gas according to the octet rule. The word valence should be somewhere in your answer.
Draw a mini periodic table and include the charges of each group in the space below. Explain why oxygen is a fairly reactive element while neon is not. So oxygen will have to share or steal electrons from other elements reactive.
Explain why beryllium loses electrons when forming ionic bonds while sulfur gains electrons. Start studying ioniccovalent compounds. Up to 24 cash back Explain why oxygen is a fairly reactive element while neon is not.
In neon the outermost orbital p is completely filled while in oxygen it. Element of Protons of Electrons of Valence Electrons Sodium Chlorine Beryllium Fluorine Lithium Oxygen Phosphorus 2. Oxygen needs to gain electrons to become stable whereas neon is a noble gas and is stable.
Neon is stable because it has 8 valence electrons. 5 Explain why beryllium loses electrons when forming ionic bonds while. 5 Explain why beryllium loses electrons when forming ionic bonds while sulfur.
Oxygen is a member of the chalcogen group on the periodic table and is a highly reactive nonmetallic element. What elements does Oxygen react with. Platinum is placed below gold in the reactivity series.
Due to presence of unpaired electrons in outermost shell of oxygen oxygen is a fairly reactive element while neon is not. In this manner Why is hydrogen the most reactive element. Answers 1 Oxygen is very reactive with Alkali metals.
Up to 24 cash back 4 Explain why oxygen is a fairly reactive element while neon is not. Just an outline of the table is all you need. Is Neon non-reactive gas.
Oxygen has 6 valence electrons and needs two to become stable. Since Neon has its outer most shell completely filled so it does not require to react with any other element in order to exchange the electron. Explain why does beryllium loses electrons when forming ionic bonds while sulfur gains electrons.
Oxygen is reactive because it needs to gain two more electrons to fill its outermost shell while neons outermost shell is completely. While oxygen has to gain 2 electrons in order to get stable. Answer 1 of 10.
Why is oxygen a fairly reactive element while neon is not. Up to 24 cash back Explain why oxygen is a fairly reactive element while neon is not. 4 Explain why oxygen is a fairly reactive element while neon is not.
Predict its reaction with dilute acids and explain your answer. As such it readily forms compounds notably oxides with almost all other elements. Lets look at the electronic configuration of both the elements.
Because it is easier for beryllium to lose two electrons than to gain six. Oxygen wants to gain electrons to become like the nearest noble gas according to the octet rule. Explain why beryllium loses electrons when forming ionic bonds while sulfur gains electrons.
Why Is Oxygen More Reactive Than Nitrogen Quora

Is Neon A Reactive Element Quora

Magnesium Description Properties Compounds Britannica

Alkaline Earth Metal Properties List Reactivity Britannica

Elements And Bonding Worksheet Elements And Bonding Worksheet 1 Classify Each Of The Following Elements As An Alkali Metal An Alkalineearth Metal Course Hero
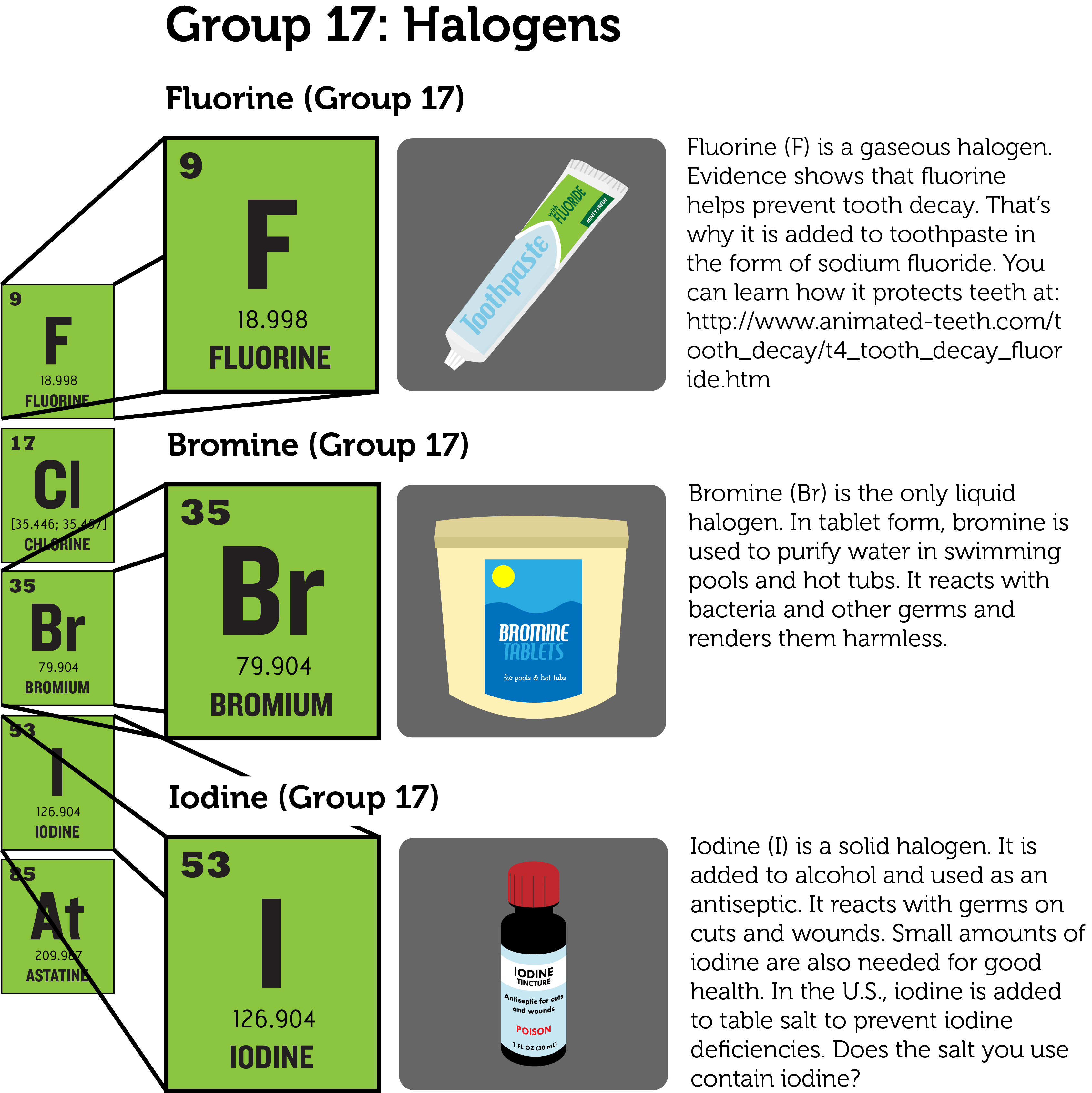
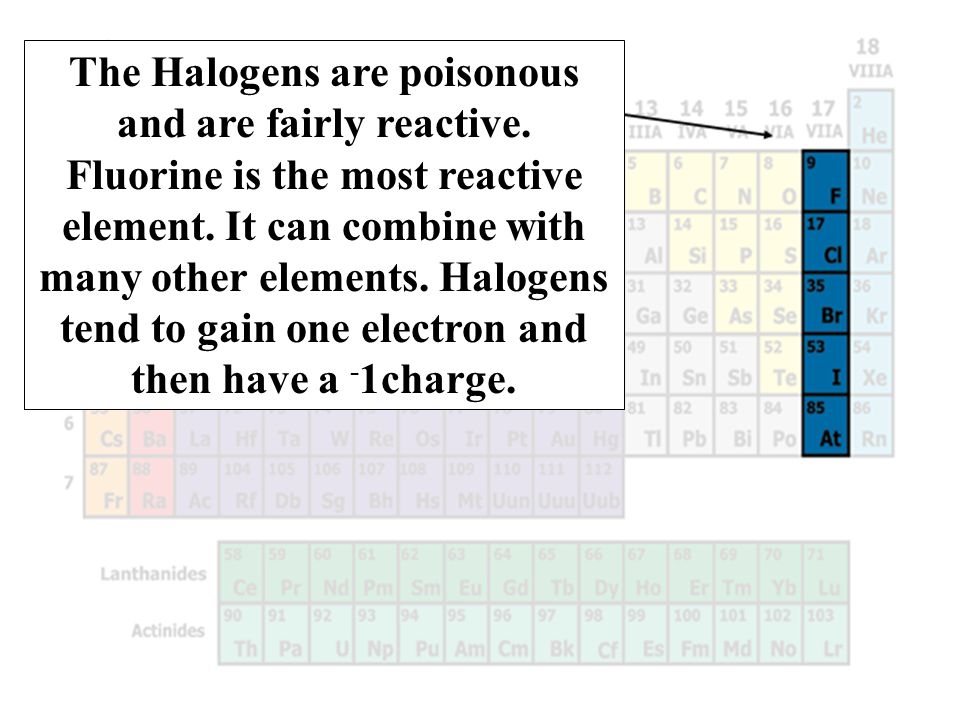
Groups Of Elements Ck 12 Foundation

The Metal Reactivity Series Compound Interest
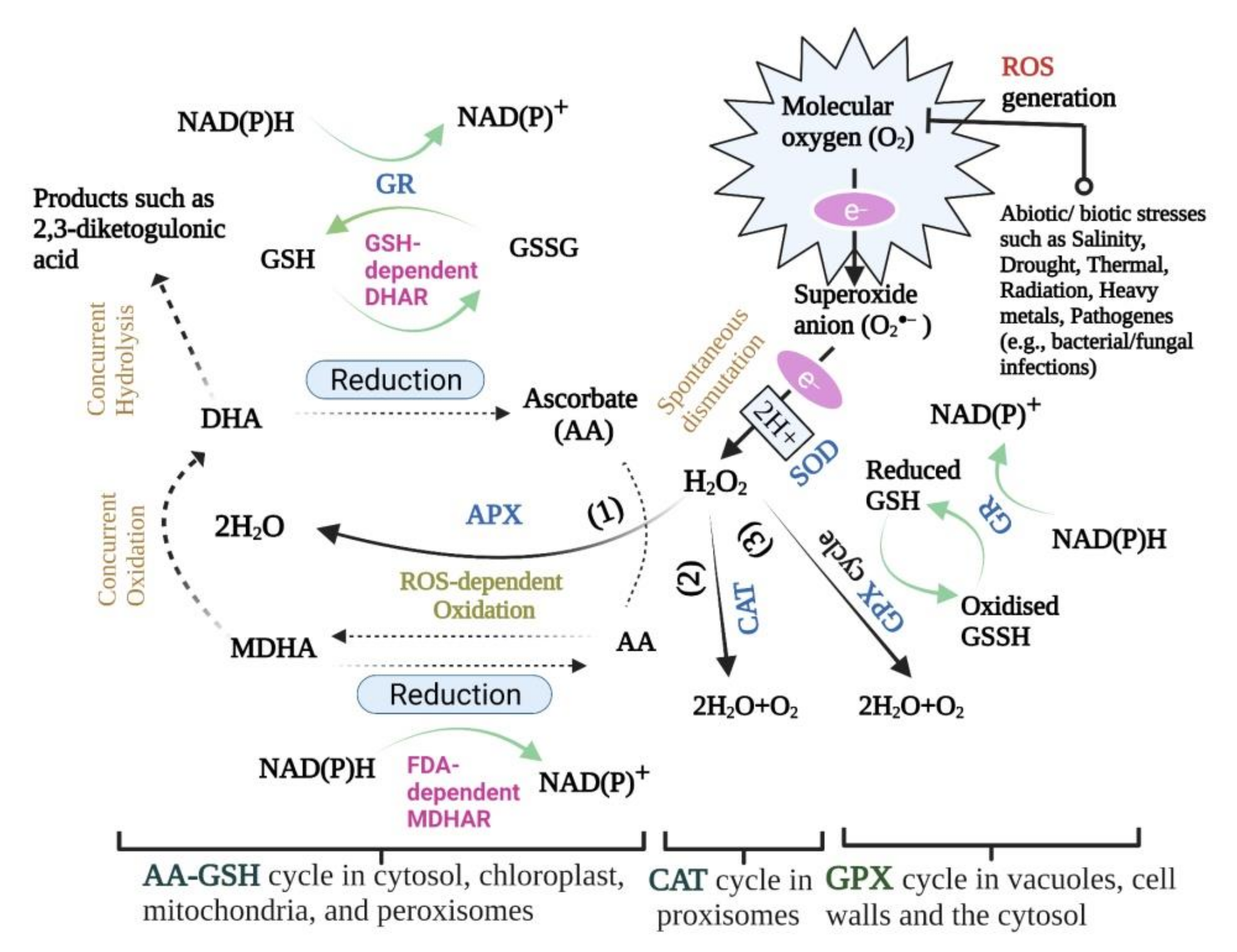
Biology Free Full Text Reactive Oxygen Species Antioxidant Responses And Implications From A Microbial Modulation Perspective Html
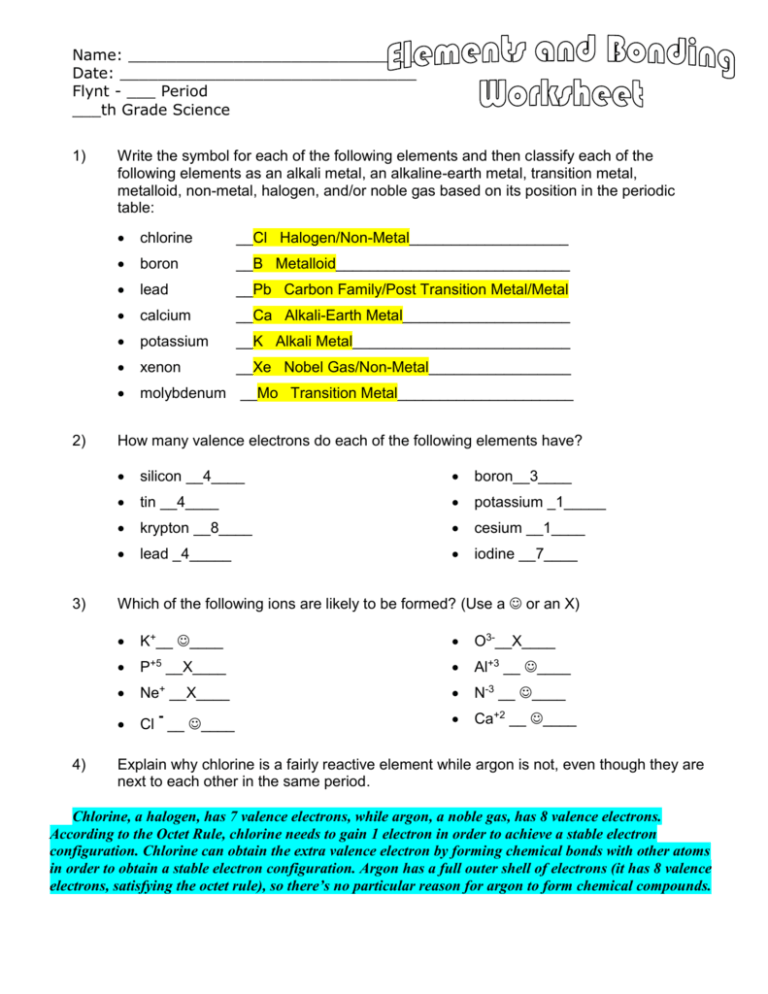
Elements And Bonding Worksheet
What Is The Least Reactive Element On The Periodic Table Of Elements Quora

Elements And Bonding Worksheet Elements And Bonding Worksheet 1 Classify Each Of The Following Elements As An Alkali Metal An Alkalineearth Metal Course Hero

Elements And Bonding Worksheet
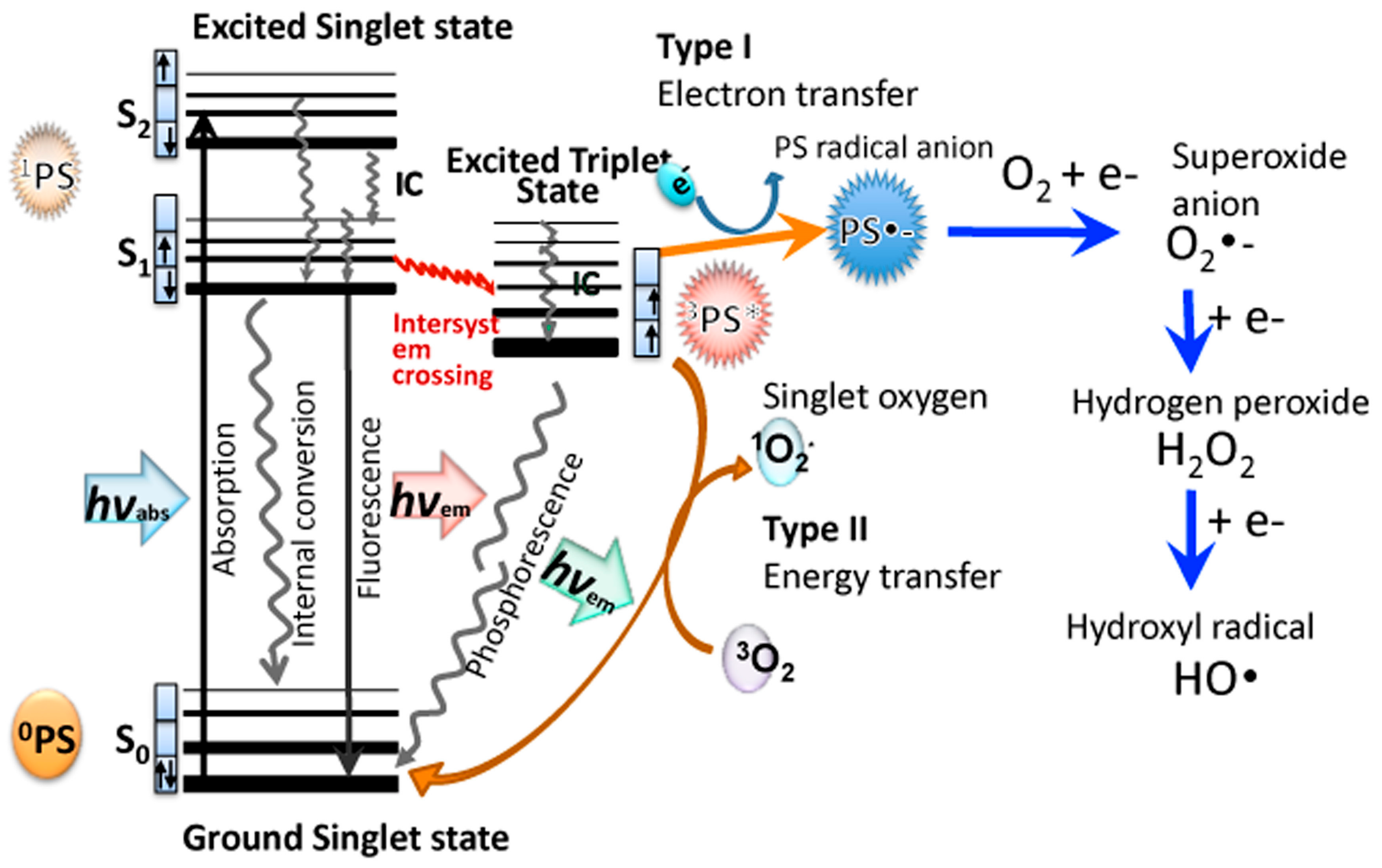
Antibiotics Free Full Text Oxygen Independent Antimicrobial Photoinactivation Type Iii Photochemical Mechanism Html

Ipc 7 D Relate The Chemical Behavior Of An Element Including Bonding To Its Placement On The Periodic Table Ppt Video Online Download

Metal Reactions Dilute Acids Water Oxygen Video Lesson Transcript Study Com
Which Nonmetals Are Among The Most Reactive Quora
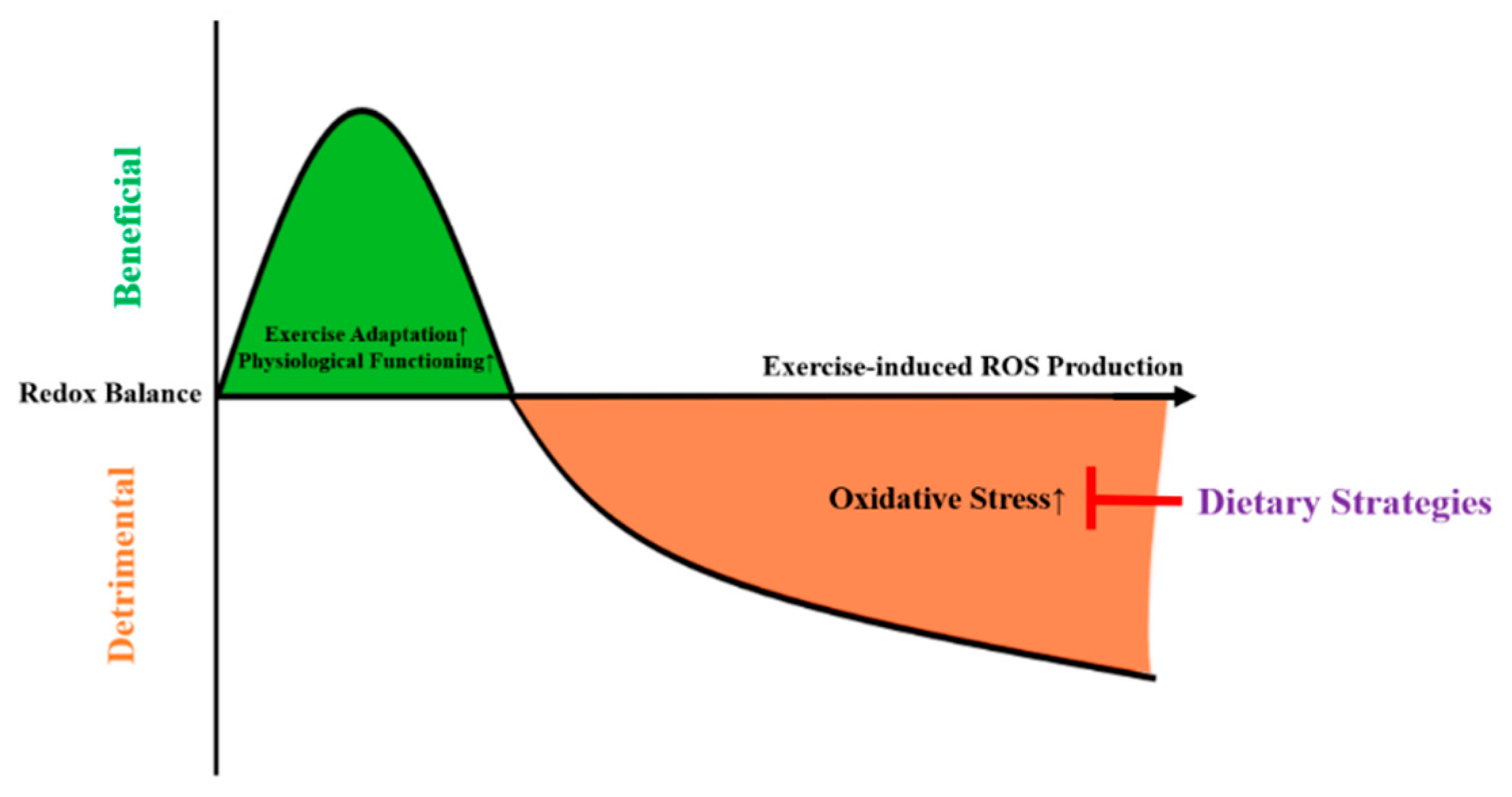
Antioxidants Free Full Text Effects Of Dietary Strategies On Exercise Induced Oxidative Stress A Narrative Review Of Human Studies Html
Comments
Post a Comment